Magnesium Carbonate: A Closer Look at a Quietly Essential Compound
Historical Development
Magnesium carbonate has quietly carried a big load since the days of early alchemists. In fact, records trace its handling to the early 18th century, when the element magnesium first got its name from Magnesia in Greece. For centuries, people kept finding new reasons to value this mineral, not only as a natural deposit—think dolomite and magnesite—but also for its transformative potential once processed. Through the Industrial Revolution, demand for lighter, pure forms picked up as factories wanted more efficient insulation, fireproofing, and food additives. Anybody who’s been around athletes or rock climbers has certainly felt its signature chalky grit, though its paths into chemistry labs, pharmaceuticals, and building materials quietly stretch back generations. It's not showy, but it always finds its way into the story when someone builds a bridge, presses pills, or creates a piece of fine porcelain.
Product Overview
Magnesium carbonate comes in a few familiar forms, with light and heavy variants showing up in lab supply catalogs, gym chalk buckets, or antacid tablets. Most commercial grades appear as a fine, white powder that’s fluffy, almost snowlike, with the lighter grades favored when bulk and softness matter and denser, granulated heavy types better suited for rigid ceramics and refractory bricks. The food industry leans on its E504 additive code, where it stabilizes flours or bulks up supplements, making the jump from raw earth to a finished product that slips into our daily routines. Those rigid forms that keep heat at bay in kilns show the versatility that’s made magnesium carbonate an industry staple—never flashy, but always present.
Physical & Chemical Properties
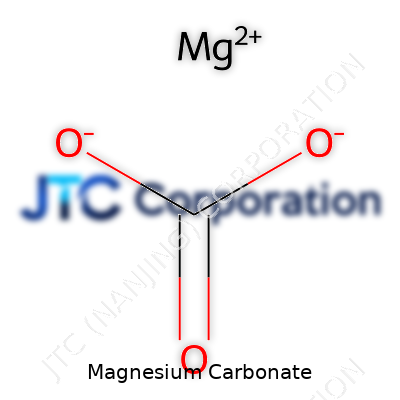
Physically, magnesium carbonate tells a familiar story among alkaline earth salts: not very soluble in water, though it reacts easily to acids with that classic fizz from escaping carbon dioxide. At room temperature, it feels soft and light, with a melting point high enough to shrug off most industrial heat processes. Its chemical formula—MgCO3—hides a backbone that’s both stable and eager to adapt. It resists dissolving in plain water, so those using it for chalk or antacid aren’t chasing clouds of dust, yet the compound changes in the presence of even weak acids, breaking down into magnesium salts and carbon dioxide. This property gives it a role both as a pH buffer and a gentle source of magnesium ions in supplements. Some forms, like nesquehonite or hydromagnesite, come with varying amounts of water bound up, leading to differences in density and handling in the lab.
Technical Specifications & Labeling
Labels for magnesium carbonate products read a bit like a technical map: purity ranges from pharmaceutical (upwards of 98%) to industrial grades, with traces of calcium or iron flagged not as defects but as background minerals. The particle size sharply affects where a lot ends up—extra fine for tablets and powdered foods, coarser granules for ceramics or construction. Moisture content also gets reported, linked to claims about how the material flows or cakes. On product sheets, you’ll see value ranges for loss on ignition, reflecting how much water or carbon dioxide escapes with heating. Batch traceability, detailed hazard phrasing, and compliance with food or pharmacopeial codes round out the small print, tying every drum or package back to strict safety and quality standards that protect users and downstream applications.
Preparation Method
The most direct way to make magnesium carbonate involves reacting a magnesium-containing solution—think magnesium chloride or sulfate—with sodium carbonate. Pour those together, and a white, cloudlike precipitate of magnesium carbonate settles out. Large-scale plants use this method, then wash, filter, and dry the solid, often tuning the process to control density or crystal size. Some legacy producers still rely on mining natural magnesite or dolomite, then purifying the ore to remove silica and iron, followed by calcination for high-purity grades. Whether chemical or mined, the aim stays clear: get clean, predictable, and safe material into users' hands with minimum fuss and maximum reliability. Every tweak in process leaves its mark on the finished product, shaping how it performs everywhere from antacids to climbing gyms.
Chemical Reactions & Modifications
Magnesium carbonate doesn’t mind reacting with acids—a simple squeeze of lemon juice on gym chalk showcases its readiness to release a brisk wave of carbon dioxide and create soluble magnesium salts. Heat changes the story again, as high temperatures drive off carbon dioxide, leaving magnesium oxide. This transformation, critical in refractory and construction materials, turns a stable, white powder into a hard, heat-tolerant substance at the heart of kilns and flame-resistant linings. In a pharmaceutical context, it soaks up acidic excipients or provides a buffered base for sensitive mixtures. Chemically, it's not invincible—strong bases and acids poke at its structure—but its resistance gives it a long working life in harsh environments, making each batch of customized magnesium carbonate a quiet but crucial anchor for many processes.
Synonyms & Product Names
In the wild world of commerce and regulation, magnesium carbonate wears plenty of masks. Chemical catalogs list it as magnesite, hydromagnesite, or dypingite depending on water content and crystal structure. Food labels usually choose E504, while the supplement aisle prefers phrases like “magnesium salts” or straightforward “magnesium carbonate.” Pharmacists may reach for “laxative magnesia,” and ceramics supply shops call for “light magnesia.” Those fluent in chemical synonyms won’t be caught off guard if they see basic magnesium carbonate or magnesium carbonate hydroxide printed on a spec sheet. Every name points back to the same family of compounds, keeping buyers and regulators aligned no matter which industry needs the chalk.
Safety & Operational Standards
Guidance from global standards and health agencies keeps magnesium carbonate supply chains clear of trouble. Inhaling dust in large amounts can absolutely irritate the airways, with the fine particles used for athletics especially likely to hang in the air. Guidelines cap allowable heavy metal content, and allergen labeling keeps cross-contamination in check for pharmaceutical and food uses. Safe plant operation matters—dust control, careful ventilation, and airtight packaging prevent exposure both during manufacture and downstream at the user level. Labels track the source of each shipment, and third-party testing ensures purity so that nobody faces unexpected health risks or unwanted reactions. While magnesium carbonate shows low toxicity, nobody wants to take chances when handling any powdered mineral—safety data sheets are standard reading.
Application Area
Magnesium carbonate lands everywhere from the climbing gym to medicine cabinets. Athletes using the powder for grip control probably don’t realize this same fluffy material keeps cakes from clumping and stabilizes the pH in specialty bottled water. The supplement and pharmaceutical industries use it as both an active and an excipient, with its antacid action something plenty of heartburn sufferers rely on. Ceramics and refractory makers stick with it for its ability to transition to heatproof magnesium oxide. Artists and industrial chemists continue to pick up new uses, with its role expanding into specialty polymers, corrosion inhibitors, and carbon sequestration materials. Across sectors—food, fitness, pharmacy, manufacturing—its utility beats most folks’ expectations every day.
Research & Development
The magnesium carbonate story isn’t stuck in the past. Research groups hunt for smarter synthesis methods, seeking to lower temperatures and reduce energy bills for large-scale production. Efforts to craft hybrid magnesium carbonate materials, blending it into nanocomposites for cleaner water or stronger plastics, keep popping up in academic journals. Teams working on carbon capture recognize the potential for magnesium carbonate as a safe, solid sink for greenhouse gases, while food scientists pursue more bioavailable supplement blends and gentler antacid formulas. Industrial labs tweak the crystal habit to tune everything from tablet binding ability to heat insulation. Down at the molecular level, new analytical techniques keep finding trace contaminants, leading to safer, purer batches for high-risk uses. Magnesium carbonate might strike people as old-fashioned, but in labs around the world, it always proves it can still teach us something new.
Toxicity Research
Years of toxicology studies keep magnesium carbonate solidly in the generally safe camp for human and environmental contact. Oral doses in reasonable quantities pass through the gastrointestinal tract without chaos, which is why it earns its place in over-the-counter antacid mixes and dietary magnesium supplements. High doses may bring on a laxative effect, but acute toxicity remains low, especially compared to many other industrial and pharmaceutical additives. Occupational health research keeps tabs on inhalation exposure: repeated breathing of mineral dusts, especially in poorly ventilated facilities, has shown the potential to cause mild irritation or, very rarely, small, reversible changes in lung function if chronic exposure goes unchecked. Studies in aquatic and soil ecosystems turn up little environmental persistence or bioaccumulation, reflecting its roots as a naturally occurring mineral. Even so, regulations hold the line on permissible exposure in workplaces, with good ventilation and dust minimization always seen as worthwhile.
Future Prospects
Magnesium carbonate’s future looks busy. Pressure to cut carbon emissions puts a bright spotlight on materials that lock away CO2 efficiently and safely, making this compound an easy pitch for new carbon capture and utilization platforms. The push for vegan and allergen-free food ingredients has natural sources of magnesium running in high gear, feeding an expanding global supplement market. Outside the lab, growing demand for safer construction and fireproof materials draws architects to its heat-resistant strengths, and new projects use magnesium carbonate as a platform for innovative, cleaner ceramics or lightweight, eco-friendly polymers. Both established and new manufacturers work to lower environmental footprints, with green chemistry techniques turning out lighter, purer, and more environmentally friendly forms. The thread running through it all is straightforward: as industries chase sustainability, magnesium carbonate proves its old reputation for adaptability is as relevant now as it was centuries ago.
Everyday Uses That Might Surprise You
Magnesium carbonate always seemed like one of those things I’d only find in a chemistry set, but it’s woven through daily life a lot more than people think. The most obvious place you’ll encounter it? At the gym. If you see a climber dusting their hands or a gymnast prepping for a routine, that fluffy white substance is almost always magnesium carbonate. They're not trying to look cool—they’re making sure sweat doesn’t get in the way. This stuff soaks up moisture fast and helps with grip when the stakes are high and palms are slippery.
Magnesium carbonate doesn’t just make athletes’ lives better. Walk down the aisle in any drugstore, and you’re likely to spot it on ingredient lists. Pharmaceutical companies use it to keep tablets from caking up and to get accurate dosages in each pill. Its ability to act as a “flow agent” keeps things smooth on the production line and safe for consumers. Antacids also rely on magnesium carbonate since it helps neutralize stomach acid. I remember my grandmother swearing by those chalky tablets after a spicy meal—they work because of magnesium carbonate doing chemistry in your gut.
Beyond the Gym and Medicine Cabinet
The world of personal care and cosmetics counts on magnesium carbonate, too. Products like face powders often include a pinch to absorb oil and keep skin looking matte throughout a long day. Toothpaste makers blend it in for mild abrasiveness, helping clean teeth without wearing them down. Even the food industry gets in on the action: restaurants and commercial kitchens sometimes add magnesium carbonate to table salt as an anti-caking agent. Lumpy salt in a shaker can ruin dinner service, so they use it to keep things flowing.
Manufacturers handling paper, ceramics, and glass also lean heavily on magnesium carbonate. In ceramics, it helps adjust the way glazes melt and set. I once visited a pottery studio where the owner explained that the whiteness and smoothness of certain tiles come from magnesium carbonate blended into the mix. It shocked me how many artists had their own “secret recipe” that included it.
Possible Risks and Smart Solutions
No chemical gets a free pass, and magnesium carbonate is no exception. Most people won’t have a problem with small doses in food or in the air at a gym, but large quantities—especially in supplements—can mess with digestion and cause an upset stomach. The FDA lists it as generally safe, but that stands only if companies stick to proper guidelines. Too much, too often, or mixed into products without careful oversight could bring trouble. Good labeling on supplements always matters. Overuse in antacids might even cause imbalances in body minerals if people rely on them more than they should. In commercial spaces, proper dust extraction protects workers from breathing in too much fine powder.
Finding Balance in Everyday Life
Magnesium carbonate shows up in places that often go unnoticed, but the benefits trickle down to nearly everyone. Its grip-enhancing power, moisture absorption, and versatility help out athletes, cooks, artists, and families. Transparency and clear safety standards give people confidence—whether they’re taking medicine, climbing a wall, or just shaking salt on dinner.
A Closer Look At Magnesium Carbonate In Everyday Foods
Some folks notice magnesium carbonate in ingredients lists and get a little worried. The name isn’t exactly something you’d find in grandma’s kitchen, right? I remember spotting it once while reading the label on a chewy mint candy. Instead of putting it back on the shelf, curiosity won—I dug in to figure out just what’s going on with this powdery substance.
Why Magnesium Carbonate Pops Up So Often
Magnesium carbonate comes from minerals and usually looks like a fine, white powder. You’ll often see it in baking powders, some medicines, even sports supplements. The reason manufacturers lean on it so much really boils down to its ability to soak up moisture and keep clumps away. In the food world, any additive that keeps salt and sugar pouring smoothly is worth its weight.
Is Eating Magnesium Carbonate Risky?
The big concern: Is it actually safe to have in your snacks or supplements? Turning to respected bodies like the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), both label magnesium carbonate as “Generally Recognized as Safe” (GRAS). Both agencies have sifted through strong research. For healthy adults, the body can handle small amounts without breaking a sweat. Most folks eat way less than the recommended upper intake of magnesium, even with foods containing it as an additive.
Stories float around on social media about so-called “hidden dangers” of ingredients that sound industrial or chemical. Fact checks matter here. Magnesium is an element your body actually needs. It plays a role in bones, nerves, muscles, and even heartbeat. Sure, you get most of your daily magnesium from leafy greens, nuts, and grains, not from sprinkled additives. Even so, the dose you’d get from food-grade additives is tiny. I asked a registered dietitian years ago at a community event, and she shrugged: “Unless you’re eating it by the spoonful, it isn’t a risk.”
What To Watch For: Digestive Upset And Special Cases
No story is one-size-fits-all. Some people, especially with certain kidney issues or digestive sensitivities, might notice upset stomachs or changes in bathroom trips if they take large supplements containing magnesium carbonate. In my own family, we stick with balanced diets and steer clear of overdoing any supplement unless a doctor says otherwise.
If you already have kidney problems, magnesium carbonate can build up—so health professionals want to know about any supplements or unusual diets. Anyone thinking about it for acid reflux, for example, should talk to a doctor. Getting more than you need from pills or powders can push the body past its comfort zone.
Keeping Food Safe And Simple
Food safety means making sure every additive serves a real purpose and gets checked for safety again and again. Personally, I stick to whole foods but don’t panic over the occasional anticaking agent on a label—especially knowing regulatory agencies have strict cutoffs before any ingredient lands on store shelves. If you care about cutting back, there’s always the option to pick less-processed foods or research brands with short ingredients lists. More than anything, being an informed eater beats worry or confusion every day of the week.
Magnesium Supplements: Not Always Trouble-Free
Some folks take magnesium carbonate as an antacid or a supplement because magnesium helps keep nerves and muscles working well. People with low magnesium levels sometimes look to tablets or powders to fill the gap. I’ve seen friends reach for these supplements after a heavy meal or during bouts of muscle cramps. Doctors even recommend magnesium in some cases. But there’s more to this mineral than most realize, especially if you overlook how much your body needs or mix it with other medicines.
Common Side Effects You Can’t Ignore
A lot of people notice stomach issues after taking magnesium carbonate. Diarrhea tends to show up most, especially if the dose runs high. It seems like your stomach wants to get rid of the extra magnesium all at once. Nausea sometimes tags along, making folks feel queasy or gassy. I remember trying a supplement with magnesium carbonate after a workout and ending up spending too much time in the bathroom.
Some notice bloating or abdominal cramps. This happens because magnesium carbonate pulls water into the intestines, which can speed up how fast everything moves through your gut. That flush can feel rough, especially for people who already deal with digestive troubles, like IBS or frequent heartburn.
Unexpected Problems You Might Not Expect
Getting too much magnesium isn’t just about an upset stomach. People with kidney problems face real risks because their bodies struggle to remove the extra mineral. That leads to a build-up, which can cause irregular heartbeats, low blood pressure, trouble breathing, or even confusion. The U.S. National Institutes of Health points out that magnesium toxicity, while rare for folks with healthy kidneys, becomes a real concern for older adults and anyone with chronic kidney disease.
Some medicines don’t mix well with magnesium carbonate. For example, antibiotics like tetracycline and some osteoporosis medications may not work as well if you take magnesium too close together. The mineral can block your body from absorbing the medicine. Every pharmacist I’ve spoken to reminds patients to space out these drugs and supplements by a few hours.
Why Dose and Timing Matter
Magnesium matters, no question. But the way people reach for supplements instead of getting it from food surprised me. Most leafy greens, nuts, seeds, and whole grains offer safe sources of this mineral. The U.S. Food and Nutrition Board suggests that most men and women can meet their needs through diet if they eat a variety of foods. Taking more seems like a shortcut, but the risks add up quickly, especially for those already healthy enough to get by without the extra pills or powders.
Practical Tips for Staying Safe
Check with a doctor before starting magnesium carbonate, especially if you deal with kidney issues or take medicines that might clash with supplements. Stick to the dose on the label or as prescribed. Most of all, listen to your body. If something feels off after taking magnesium, don’t ignore it. Stomach trouble, weakness, or confusion signals it’s time to stop and talk to a medical professional. It pays to read up, ask questions, and treat your body with the respect it deserves.
What Happens When You Store It Wrong
Magnesium carbonate helps a lot of people—from athletes fighting sweaty palms to folks making medicine or food. But things go south fast if the storage game isn’t on point. Leave magnesium carbonate open to moist air or let it sit in a humid corner, it starts soaking up water. The result? The powder clumps, loses its fine texture, and for certain uses, its usefulness drops off. I’ve seen food storerooms where sacks turned to solid blocks just because someone parked them beside a leaky pipe. Nobody wants to grind apart what should pour out like flour.
Simple Enemies: Water, Air, and Heat
It’s hard to fight what you can’t see. Humidity works silently—it sneaks in and causes magnesium carbonate to absorb moisture, then caking and breaking it down. Oxygen isn't a massive issue here, but dust and other airborne bits can land in bags or open bins and mess with the product. Heat speeds up those reactions, so stashing this powder next to radiators or sunlit windows doesn't make sense.
Get Storage Right from the Start
Trying to save space or money with cheap containers rarely works out. The best practice is always grabbing airtight and moisture-proof containers. Think thick plastic tubs or metal cans with a rubber seal. Not every household or business wants to shell out for specialty packaging, but it pays back every time you open a tub and the powder pours just as smoothly as day one.
Once the right container is sorted, location matters. Pick a spot out of direct sunlight. Ideally, the shelf lives in a cool, dry area. Basements and garages often feel like the obvious answer, but they usually bring too much dampness. I’ve seen pharmacies use silica gel packs in storage tubs during sticky summers—it’s a simple hack, but it prevents headaches and expensive waste.
Safe and Clean Handling Every Time
All those gym chalk bins or bulk ingredient jars need to keep contamination low. Scoops or hands fresh from washing, lids closed between uses—pretty basic steps, but worth their weight in product savings. Reusing containers without cleaning may bring unwanted moisture in, so a dry wipe down every refill is worth the effort.
Personal safety doesn’t hurt, either. No one wants to breathe in dust clouds, so handle the powder gently and keep the lid handy. Even in commercial kitchens, a little care with each opening keeps both workers and the magnesium carbonate safe.
Learning from Mistakes
Once, a batch of magnesium carbonate stored in carelessly tied plastic bags ruined an entire batch of baked goods. Moisture made the powder inconsistent, baking results went haywire, and money went down the drain. Since then, we’ve used bins with snapping lids, and not a single ruined cake since. Storage isn’t glamorous, but it quietly saves money and stress—one dry scoop at a time.
Wrapping Up the Basics
For homes, gyms, bakeries, or labs, dry containers kept far from moisture and heat solve most storage problems for magnesium carbonate. This simple step keeps waste down, cuts costs, and ensures the powder does its job wherever it lands. Good storage pays, and good habits stick around longer than any bag of powder ever will.
The Rise of Magnesium Talk
Magnesium supplements get more shelf space every year. There’s a reason behind this: many people simply don’t get enough magnesium through diet alone. Folks dealing with stress, athletes sweating buckets, and people wanting stronger bones all look toward magnesium for support. Not every form of magnesium works the same in the body, though, and magnesium carbonate stands out for a few reasons worth discussing.
What’s Special About Magnesium Carbonate?
Magnesium carbonate shows up in both medicine cabinets and gym chalk bags. In supplement form, it’s a white powder that, once inside the stomach, reacts with acid and turns into magnesium chloride. This reaction makes it more absorbable, though it’s not as quickly available as forms like magnesium citrate. Still, magnesium carbonate provides a solid option for those seeking to build up magnesium stores.
Digestive Effects and Stomach Sensitivity
Anyone who’s ever taken a raw magnesium supplement knows the digestive system can push back. Magnesium carbonate tends to cause less diarrhea or stomach upset compared to some forms like magnesium oxide or citrate. That milder touch on the gut comes from its slower reaction in the digestive tract. Some people may still experience bloating if they have particularly sensitive systems or take larger amounts. Starting with smaller doses ends up suiting most people better.
Why Low Magnesium Sneaks Up on People
Busy routines and food choices play a big role in modern magnesium levels. Diets heavy on processed grains, minimal in nuts and greens, leave folks short. Research from the National Institutes of Health shows nearly half of Americans don’t hit the recommended daily intake. Signs of low magnesium often look like fatigue, restless legs, or muscle cramps. Ignoring these signals means missing a chance to fix something simple.
Is Supplementing Needed?
Supplements can help close the gap for those with confirmed low magnesium. The Centers for Disease Control links steady magnesium levels to heart health, steady blood sugars, and bone maintenance. A supplement like magnesium carbonate works best in people with mild deficiencies or those who cannot eat certain foods because of allergies or restricted diets. People on certain medications, such as diuretics or proton pump inhibitors, may find themselves needing extra magnesium under medical guidance.
Quality and Purity Matter
Not all supplements deliver on label promises. Products from reputable companies provide third-party test results and stick to transparent sourcing. Trusted brands care about where they get their raw minerals and whether they contain heavy metals or undisclosed fillers. Customers should always look for supplements that give full disclosure on these quality checks.
Safe Practices and Dosing
Getting enough magnesium from food remains best for most people. Spinach, almonds, and pumpkin seeds naturally supply the mineral without side effects. Those wanting to try supplements should always check with a healthcare professional, especially individuals managing kidney disease or taking medications affecting mineral balance. Going above the recommended dose might backfire, leading to diarrhea or even worsening kidney function in susceptible people.
Better Habits and Smarter Choices
Building a healthy relationship with minerals starts at the grocery store. Focusing on whole foods, adding nuts, and tasting new leafy greens go further than most pills. If a supplement is needed, magnesium carbonate serves as an option, but the best results come with good advice, reliable products, and steady routines. Supplements fill in gaps; they don’t take the place of a balanced plate.